what new information did antoine lavoisier contribute to the understanding of the atom
| Antoine-Laurent de Lavoisier | |
|---|---|
 Portrait of Antoine-Laurent Lavoisier and his Married woman by Jacques-Louis David (detail) | |
| Built-in | (1743-08-26)26 August 1743 Paris, France |
| Died | eight May 1794(1794-05-08) (aged 50) Paris, France |
| Crusade of death | Execution by guillotine |
| Resting place | Catacombs of Paris |
| Alma mater | Collège des Quatre-Nations, Academy of Paris |
| Known for |
|
| Spouse(south) | Marie-Anne Paulze Lavoisier (married 1771–1794) |
| Scientific career | |
| Fields | Biologist, chemist |
| Notable students | Éleuthère Irénée du Pont |
| Influences | Guillaume-François Rouelle, Étienne Condillac |
| Signature | |
 | |
Antoine-Laurent de Lavoisier (French: [ɑ̃twan lɔʁɑ̃ də lavwazje] lav-WUZ-ee-ay,[one] lə-VWAH-zee-ay;[2] [3] 26 August 1743 – 8 May 1794),[4] also Antoine Lavoisier after the French Revolution, was a French nobleman and chemist who was fundamental to the 18th-century chemical revolution and who had a large influence on both the history of chemical science and the history of biology.[v] Information technology is mostly accepted that Lavoisier's great accomplishments in chemistry stem largely from his changing the science from a qualitative to a quantitative one. Lavoisier is well-nigh noted for his discovery of the part oxygen plays in combustion. He recognized and named oxygen (1778) and hydrogen (1783), and opposed the phlogiston theory. Lavoisier helped construct the metric system, wrote the commencement extensive listing of elements, and helped to reform chemical nomenclature. He predicted the existence of silicon (1787)[6] and discovered that, although matter may change its course or shape, its mass ever remains the same.
Lavoisier was a powerful fellow member of a number of aloof councils, and an administrator of the Ferme générale. The Ferme générale was i of the near hated components of the Ancien Régime considering of the profits it took at the expense of the state, the secrecy of the terms of its contracts, and the violence of its armed agents.[7] All of these political and economic activities enabled him to fund his scientific research. At the height of the French Revolution, he was charged with taxation fraud and selling adulterated tobacco, and was guillotined.
Biography

Early life and education
Antoine-Laurent Lavoisier was born to a wealthy family of the nobility in Paris on 26 August 1743. The son of an chaser at the Parlement of Paris, he inherited a big fortune at the historic period of five upon the death of his mother.[8] Lavoisier began his schooling at the Collège des Quatre-Nations, University of Paris (also known every bit the Collège Mazarin) in Paris in 1754 at the age of 11. In his last 2 years (1760–1761) at the school, his scientific interests were aroused, and he studied chemistry, botany, astronomy, and mathematics. In the philosophy grade he came under the tutelage of Abbé Nicolas Louis de Lacaille, a distinguished mathematician and observational astronomer who imbued the young Lavoisier with an involvement in meteorological ascertainment, an enthusiasm which never left him. Lavoisier entered the school of police force, where he received a bachelor'due south degree in 1763 and a licentiate in 1764. Lavoisier received a constabulary degree and was admitted to the bar, but never proficient as a lawyer. However, he connected his scientific education in his spare time.
Early scientific work
Lavoisier's teaching was filled with the ideals of the French Enlightenment of the time, and he was fascinated past Pierre Macquer's dictionary of chemistry. He attended lectures in the natural sciences. Lavoisier's devotion and passion for chemical science were largely influenced by Étienne Condillac, a prominent French scholar of the 18th century. His commencement chemical publication appeared in 1764. From 1763 to 1767, he studied geology under Jean-Étienne Guettard. In collaboration with Guettard, Lavoisier worked on a geological survey of Alsace-Lorraine in June 1767. In 1764 he read his first paper to the French Academy of Sciences, French republic's nearly elite scientific society, on the chemical and concrete backdrop of gypsum (hydrated calcium sulfate), and in 1766 he was awarded a gilded medal by the Rex for an essay on the problems of urban street lighting.[9] In 1768 Lavoisier received a provisional appointment to the Academy of Sciences.[10] In 1769, he worked on the first geological map of France.

Lavoisier conducting an experiment on respiration in the 1770s
Research benefitting the public expert
While Lavoisier is normally known for his contributions to the sciences, he also dedicated a meaning portion of his fortune and piece of work toward benefitting the public.[eleven] [12] [13] [xiv] Lavoisier was a humanitarian—he cared deeply near the people in his country and oftentimes concerned himself with improving the livelihood of the population by agriculture, industry, and the sciences.[12] The outset instance of this occurred in 1765, when he submitted an essay on improving urban street lighting to the French Academy of Sciences.[12] [13] [14]
Three years later in 1768, he focused on a new project to design an aqueduct. The goal was to bring h2o from the river Yvette into Paris and then that the citizens could have clean drinking water. Simply, since the construction never commenced, he instead turned his focus to purifying the water from the Seine. This was the project that interested Lavoisier in the chemical science of water and public sanitation duties.[fourteen]
Additionally, he was interested in air quality and spent some time studying the wellness risks associated with gunpowder'south upshot on the air.[13] In 1772, he performed a study on how to reconstruct the Hôtel-Dieu hospital, later on information technology had been damaged by fire, in a way that would permit proper ventilation and clean air throughout.[14]
At the time, the prisons in Paris were known to be largely unlivable and the prisoners' handling inhumane.[eleven] Lavoisier took part in investigations in 1780 (and once more in 1791) on the hygiene in prisons and had made suggestions to amend living conditions, suggestions which were largely ignored.[11] [xiv]
In one case a part of the University, Lavoisier also held his own competitions to button the direction of research towards bettering the public and his own work.[13] I such project he proposed in 1793 was to better public wellness on the "insalubrious arts".
Lavoisier had a vision of public education having roots in "scientific sociability" and philanthropy.[13]
Lavoisier gained a vast majority of his income through buying stock in the General Farm, which immune him to work on scientific discipline total-time, live comfortably, and allowed him to contribute financially to better the customs.[14] (It would also contribute to his demise during the Reign of Terror many years afterward.[15])
It was very difficult to secure public funding for the sciences at the time, and additionally non very financially profitable for the average scientist, so Lavoisier used his wealth to open a very expensive and sophisticated laboratory in France so that aspiring scientists could study without the barriers of securing funding for their research.[eleven] [14]
He also pushed for public instruction in the sciences. He founded 2 organizations, Lycée [fr] and Musée des Arts et Métiers, which were created to serve equally educational tools for the public. Funded by the wealthy and noble, the Lycée regularly taught courses to the public beginning in 1793.[13]
Ferme générale and union

Portrait of Lavoisier explaining to his wife the result of his experiments on air by Ernest Board
At the age of 26, effectually the fourth dimension he was elected to the Academy of Sciences, Lavoisier bought a share in the Ferme générale, a tax farming financial visitor which advanced the estimated revenue enhancement revenue to the majestic authorities in return for the right to collect the taxes. On behalf of the Ferme générale Lavoisier commissioned the edifice of a wall around Paris so that customs duties could be collected from those transporting goods into and out of the metropolis.[xvi] His participation in the collection of its taxes did not help his reputation when the Reign of Terror began in France, as taxes and poor government reform were the principal motivators during the French Revolution.
Lavoisier consolidated his social and economic position when, in 1771 at historic period 28, he married Marie-Anne Pierrette Paulze, the thirteen-year-former girl of a senior member of the Ferme générale.[4] She was to play an of import function in Lavoisier'due south scientific career—notably, she translated English documents for him, including Richard Kirwan'due south Essay on Phlogiston and Joseph Priestley's inquiry. In addition, she assisted him in the laboratory and created many sketches and carved engravings of the laboratory instruments used by Lavoisier and his colleagues for their scientific works. Madame Lavoisier edited and published Antoine's memoirs (whether any English translations of those memoirs accept survived is unknown every bit of today) and hosted parties at which eminent scientists discussed ideas and issues related to chemistry.[17]
A portrait of Antoine and Marie-Anne Lavoisier was painted by the famed artist Jacques-Louis David. Completed in 1788 on the eve of the Revolution, the painting was denied a customary public display at the Paris Salon for fright that information technology might inflame anti-aristocratic passions.[18]
For three years following his entry into the Ferme générale, Lavoisier's scientific activity diminished somewhat, for much of his time was taken upwards with official Ferme générale business. He did, however, present one important memoir to the Academy of Sciences during this period, on the supposed conversion of water into globe by evaporation. Past a very precise quantitative experiment, Lavoisier showed that the "earthy" sediment produced after long-continued reflux heating of h2o in a drinking glass vessel was not due to a conversion of the water into earth merely rather to the gradual disintegration of the within of the glass vessel produced by the humid water. He besides attempted to introduce reforms in the French budgetary and revenue enhancement organisation to help the peasants.
Adulteration of tobacco
The Farmers Full general held a monopoly of the product, import and sale of tobacco in France, and the taxes they levied on tobacco brought revenues of thirty million livres a year. This revenue began to autumn considering of a growing blackness market place in tobacco that was smuggled and adulterated, most commonly with ash and water. Lavoisier devised a method of checking whether ash had been mixed in with tobacco: "When a spirit of vitriol, aqua fortis or another acrid solution is poured on ash, there is an immediate very intense effervescent reaction, accompanied by an easily detected noise." Lavoisier besides noticed that the addition of a small corporeality of ash improved the flavor of tobacco. Of one vendor selling adulterated goods, he wrote "His tobacco enjoys a very adept reputation in the province... the very small proportion of ash that is added gives it a particularly pungent flavour that consumers look for. Perhaps the Farm could gain some advantage past adding a bit of this liquid mixture when the tobacco is made." Lavoisier also establish that while calculation a lot of water to bulk the tobacco up would cause it to ferment and scent bad, the add-on of a very small corporeality improved the product. Thereafter the factories of the Farmers General added, equally he recommended, a consistent vi.three% of water by volume to the tobacco they processed.[19] To allow for this addition, the Farmers General delivered to retailers seventeen ounces of tobacco while just charging for sixteen.[20] To ensure that only these authorised amounts were added, and to exclude the black market, Lavoisier saw to it that a watertight arrangement of checks, accounts, supervision and testing fabricated it very hard for retailers to source contraband tobacco or to better their profits by bulking it up. He was energetic and rigorous in implementing this, and the systems he introduced were securely unpopular with the tobacco retailers across the country. This unpopularity was to have consequences for him during the French Revolution.[21]
Royal Committee on Agriculture
Lavoisier urged the establishment of a Royal Commission on Agriculture. He then served as its Secretarial assistant and spent considerable sums of his ain money in order to improve the agricultural yields in the Sologne, an area where farmland was of poor quality. The humidity of the region ofttimes led to a blight of the rye harvest, causing outbreaks of ergotism amid the population. In 1788 Lavoisier presented a written report to the Commission detailing ten years of efforts on his experimental farm to innovate new crops and types of livestock. His conclusion was that despite the possibilities of agricultural reforms, the tax arrangement left tenant farmers with so little that it was unrealistic to expect them to change their traditional practices.[22]
Gunpowder Commission

Lavoisier'southward researches on combustion were carried out in the midst of a very decorated schedule of public and private duties, specially in connectedness with the Ferme Générale. At that place were also innumerable reports for and committees of the Academy of Sciences to investigate specific issues on society of the majestic government. Lavoisier, whose organizing skills were outstanding, oft landed the task of writing up such official reports. In 1775 he was fabricated one of four commissioners of gunpowder appointed to replace a individual company, similar to the Ferme Générale, which had proved unsatisfactory in supplying France with its munitions requirements. Every bit a result of his efforts, both the quantity and quality of French gunpowder greatly improved, and information technology became a source of revenue for the authorities. His date to the Gunpowder Commission brought 1 great benefit to Lavoisier'south scientific career also. As a commissioner, he enjoyed both a house and a laboratory in the Regal Arsenal. Here he lived and worked between 1775 and 1792.
Lavoisier was a determinative influence in the formation of the Du Pont gunpowder business considering he trained Éleuthère Irénée du Pont, its founder, on gunpowder-making in France; the latter said that the Du Pont gunpowder mills "would never have been started but for his kindness to me."[23] : 40
During the Revolution
In June 1791, Lavoisier fabricated a loan of 71,000 livres to Pierre Samuel du Pont de Nemours to purchase a printing works then that du Pont could publish a newspaper, La Correspondance Patriotique. The program was for this to include both reports of debates in the National Elective Associates likewise as papers from the Academy of Sciences.[24] The revolution quickly disrupted the elder du Pont's first newspaper, but his son E.I. du Pont soon launched Le Republicain and published Lavoisier'south latest chemical science texts.[23] : xv
Lavoisier also chaired the committee set up to establish a uniform arrangement of weights and measures[25] [26] which in March 1791 recommended the adoption of the metric system.[27] The new system of weights and measures was adopted by the Convention on 1 August 1793.[28] Lavoisier himself was removed from the commission on weights and measures on 23 December 1793, together with mathematician Pierre-Simon Laplace and several other members, for political reasons.[26]
One of his terminal major works was a proposal to the National Convention for the reform of French education. He also intervened on behalf of a number of strange-built-in scientists including mathematician Joseph Louis Lagrange, helping to exempt them from a mandate stripping all foreigners of possessions and liberty.[29]
Final days and execution

As the French Revolution gained momentum, attacks mounted on the deeply unpopular Ferme générale, and it was eventually abolished in March 1791.[30] In 1792 Lavoisier was forced to resign from his post on the Gunpowder Committee and to motion from his firm and laboratory at the Majestic Arsenal. On 8 August 1793, all the learned societies, including the Academy of Sciences, were suppressed at the asking of Abbé Grégoire.[28]
On 24 November 1793, the arrest of all the onetime tax farmers was ordered. Lavoisier and the other Farmers General faced nine accusations of defrauding the state of money owed to it, and of adding water to tobacco before selling it. Lavoisier drafted their defence, refuting the fiscal accusations, reminding the court of how they had maintained a consistently high quality of tobacco. The courtroom was even so inclined to believe that by condemning them and seizing their appurtenances, information technology would recover huge sums for the land.[20] Lavoisier was convicted and guillotined on viii May 1794 in Paris, at the age of l, along with his 27 co-defendants.[31]
Co-ordinate to pop legend, the appeal to spare his life then that he could go along his experiments was cutting short by the gauge, Coffinhal: "La République n'a pas besoin de savants ni de chimistes; le cours de la justice ne peut être suspendu." ("The Republic needs neither scholars nor chemists; the course of justice cannot exist delayed.")[32] The judge Coffinhal himself would be executed less than three months subsequently, in the wake of the Thermidorian reaction.
Lavoisier'south importance to science was expressed by Lagrange who lamented the beheading by maxim: "Il ne leur a fallu qu'un moment pour faire tomber cette tête, et cent années peut-être ne suffiront pas cascade en reproduire une semblable." ("It took them simply an instant to cutting off this caput, and ane hundred years might not suffice to reproduce its like.")[33] [34]
Post-mortem
A twelvemonth and a half after his execution, Lavoisier was completely exonerated past the French government. During the White Terror, his belongings were delivered to his widow. A brief note was included, reading "To the widow of Lavoisier, who was falsely convicted".[35]
About a century later on his death, a statue of Lavoisier was erected in Paris. Information technology was later discovered that the sculptor had non actually copied Lavoisier's head for the statue, but used a spare head of the Marquis de Condorcet, the Secretary of the University of Sciences during Lavoisier's final years.[ citation needed ] Lack of money prevented alterations from existence made. The statue was melted down during the Second World War and has not been replaced. I of the main "lycées" (high schools) in Paris and a street in the eighth arrondissement are named subsequently Lavoisier, and statues of him are establish on the Hôtel de Ville and on the façade of the Cour Napoléon of the Louvre. His name is i of the 72 names of eminent French scientists, engineers and mathematicians inscribed on the Eiffel Tower besides every bit on buildings effectually Killian Court at MIT in Cambridge, MA.
Contributions to chemistry
Oxygen theory of combustion

During late 1772 Lavoisier turned his attention to the phenomenon of combustion, the topic on which he was to brand his nigh meaning contribution to science. He reported the results of his first experiments on combustion in a note to the Academy on 20 October, in which he reported that when phosphorus burned, it combined with a large quantity of air to produce acid spirit of phosphorus, and that the phosphorus increased in weight on burning. In a second sealed note deposited with the Academy a few weeks later on (1 Nov) Lavoisier extended his observations and conclusions to the burning of sulfur and went on to add that "what is observed in the combustion of sulfur and phosphorus may well take identify in the instance of all substances that gain in weight past combustion and calcination: and I am persuaded that the increase in weight of metallic calces is due to the same cause."
Joseph Black's "fixed air"
During 1773 Lavoisier determined to review thoroughly the literature on air, peculiarly "fixed air," and to repeat many of the experiments of other workers in the field. He published an account of this review in 1774 in a volume entitled Opuscules physiques et chimiques (Physical and Chemical Essays). In the course of this review, he made his beginning full report of the work of Joseph Black, the Scottish chemist who had carried out a series of archetype quantitative experiments on the mild and caustic alkalies. Black had shown that the difference between a mild brine, for case, chalk (CaCO3), and the caustic form, for example, quicklime (CaO), lay in the fact that the former independent "fixed air," not common air fixed in the chalk, merely a distinct chemical species, now understood to exist carbon dioxide (COtwo), which was a constituent of the atmosphere. Lavoisier recognized that Black's fixed air was identical with the air evolved when metal calces were reduced with charcoal and even suggested that the air which combined with metals on calcination and increased the weight might be Black'southward stock-still air, that is, CO2.
Joseph Priestley

In the jump of 1774, Lavoisier carried out experiments on the calcination of can and atomic number 82 in sealed vessels, the results of which conclusively confirmed that the increase in weight of metals in combustion was due to combination with air. Only the question remained about whether information technology was in combination with common atmospheric air or with only a office of atmospheric air. In October the English chemist Joseph Priestley visited Paris, where he met Lavoisier and told him of the air which he had produced past heating the blood-red calx of mercury with a called-for glass and which had supported combustion with extreme vigor. Priestley at this time was unsure of the nature of this gas, but he felt that it was an peculiarly pure course of common air. Lavoisier carried out his own research on this peculiar substance. The consequence was his memoir On the Nature of the Principle Which Combines with Metals during Their Calcination and Increases Their Weight, read to the University on 26 April 1775 (commonly referred to as the Easter Memoir). In the original memoir, Lavoisier showed that the mercury calx was a truthful metallic calx in that information technology could exist reduced with charcoal, giving off Blackness's stock-still air in the procedure.[36] When reduced without charcoal, information technology gave off an air which supported respiration and combustion in an enhanced way. He concluded that this was simply a pure form of common air and that it was the air itself "undivided, without alteration, without decomposition" which combined with metals on calcination.
After returning from Paris, Priestley took up once again his investigation of the air from mercury calx. His results now showed that this air was not just an specially pure form of common air merely was "five or vi times improve than common air, for the purpose of respiration, inflammation, and ... every other use of common air". He chosen the air dephlogisticated air, as he thought information technology was common air deprived of its phlogiston. Since it was therefore in a country to absorb a much greater quantity of phlogiston given off by burning bodies and respiring animals, the greatly enhanced combustion of substances and the greater ease of breathing in this air were explained.
Pioneer of stoichiometry
Lavoisier's researches included some of the beginning truly quantitative chemical experiments. He carefully weighed the reactants and products of a chemic reaction in a sealed glass vessel so that no gases could escape, which was a crucial stride in the advancement of chemistry.[37] In 1774, he showed that, although thing can alter its land in a chemical reaction, the total mass of matter is the same at the end as at the beginning of every chemical change. Thus, for instance, if a piece of woods is burned to ashes, the full mass remains unchanged if gaseous reactants and products are included. Lavoisier'due south experiments supported the law of conservation of mass. In France it is taught as Lavoisier'south Law and is paraphrased from a statement in his Traité Élémentaire de Chimie: "Nothing is lost, nothing is created, everything is transformed." Mikhail Lomonosov (1711–1765) had previously expressed like ideas in 1748 and proved them in experiments; others whose ideas pre-date the work of Lavoisier include Jean Rey (1583–1645), Joseph Black (1728–1799), and Henry Cavendish (1731–1810).[38]
Chemical nomenclature
Lavoisier, together with Louis-Bernard Guyton de Morveau, Claude-Louis Berthollet, and Antoine François de Fourcroy, submitted a new program for the reforms of chemic nomenclature to the Academy in 1787, for there was virtually no rational arrangement of chemic classification at this time. This work, titled Méthode de nomenclature chimique (Method of Chemical Nomenclature, 1787), introduced a new system which was tied inextricably to Lavoisier's new oxygen theory of chemistry.[39] The Classical elements of earth, air, fire, and water were discarded, and instead some 55 substances which could not be decomposed into simpler substances by any known chemic ways were provisionally listed as elements. The elements included light; caloric (matter of heat); the principles of oxygen, hydrogen, and azote (nitrogen); carbon; sulfur; phosphorus; the yet unknown "radicals" of hydrochloric acid (hydrochloric acid), boric acrid, and "fluoric" acid; 17 metals; 5 earths (mainly oxides of all the same unknown metals such equally magnesia, baria, and strontia); three alkalies (potash, soda, and ammonia); and the "radicals" of 19 organic acids. The acids, regarded in the new organisation as compounds of various elements with oxygen, were given names which indicated the chemical element involved together with the degree of oxygenation of that element, for example sulfuric and sulfurous acids, phosphoric and phosphorous acids, nitric and nitrous acids, the "ic" termination indicating acids with a higher proportion of oxygen than those with the "ous" ending. Similarly, salts of the "ic" acids were given the terminal messages "ate," as in copper sulfate, whereas the salts of the "ous" acids terminated with the suffix "ite," as in copper sulfite. The full consequence of the new nomenclature can be gauged past comparing the new name "copper sulfate" with the old term "vitriol of Venus." Lavoisier's new nomenclature spread throughout Europe and to the United states and became common employ in the field of chemistry. This marked the beginning of the anti-phlogistic approach to the field.
Chemic revolution and opposition
Lavoisier is commonly cited as a central contributor to the chemical revolution. His precise measurements and meticulous keeping of balance sheets throughout his experiment were vital to the widespread acceptance of the law of conservation of mass. His introduction of new terminology, a binomial system modeled later on that of Linnaeus, also helps to mark the dramatic changes in the field which are referred to mostly equally the chemic revolution. Lavoisier encountered much opposition in trying to alter the field, specially from British phlogistic scientists. Joseph Priestley, Richard Kirwan, James Keir, and William Nicholson, among others, argued that quantification of substances did not imply conservation of mass.[40] Rather than reporting factual prove, opposition claimed Lavoisier was misinterpreting the implications of his enquiry. One of Lavoisier's allies, Jean Baptiste Biot, wrote of Lavoisier'southward methodology, "one felt the necessity of linking accuracy in experiments to rigor of reasoning."[xl] His opposition argued that precision in experimentation did not imply precision in inferences and reasoning. Despite opposition, Lavoisier continued to use precise instrumentation to convince other chemists of his conclusions, often results to v to viii decimal places. Nicholson, who estimated that only 3 of these decimal places were meaningful, stated:
If it be denied that these results are pretended to be truthful in the concluding figures, I must beg get out to observe, that these long rows of figures, which in some instances extend to a thousand times the nicety of experiment, serve just to exhibit a parade which true science has no need of: and, more than than this, that when the real degree of accuracy in experiments is thus hidden from our contemplation, nosotros are somewhat disposed to uncertainty whether the exactitude scrupuleuse of the experiments be indeed such equally to return the proofs de fifty'ordre demonstratif.[41]
Notable works

Easter memoir
The "official" version of Lavoisier's Easter Memoir appeared in 1778. In the intervening period, Lavoisier had ample fourth dimension to repeat some of Priestley's latest experiments and perform some new ones of his own. In improver to studying Priestley'due south dephlogisticated air, he studied more thoroughly the residue air after metals had been calcined. He showed that this balance air supported neither combustion nor respiration and that approximately five volumes of this air added to one book of the dephlogisticated air gave mutual atmospheric air. Common air was then a mixture of two distinct chemic species with quite dissimilar backdrop. Thus when the revised version of the Easter Memoir was published in 1778, Lavoisier no longer stated that the principle which combined with metals on calcination was but common air but "zilch else than the healthiest and purest part of the air" or the "eminently respirable part of the air". The same year he coined the proper noun oxygen for this constituent of the air, from the Greek words meaning "acid former".[36] [42] He was struck past the fact that the combustion products of such nonmetals as sulfur, phosphorus, charcoal, and nitrogen were acidic. He held that all acids contained oxygen and that oxygen was therefore the acidifying principle.
Dismantling phlogiston theory
Lavoisier'due south chemical research between 1772 and 1778 was largely concerned with developing his ain new theory of combustion. In 1783 he read to the university his newspaper entitled Réflexions sur le phlogistique (Reflections on Phlogiston), a full-scale assail on the current phlogiston theory of combustion. That year Lavoisier too began a series of experiments on the composition of water which were to prove an important capstone to his combustion theory and win many converts to it. Many investigators had been experimenting with the combination of Henry Cavendish's inflammable air, which Lavoisier termed hydrogen (Greek for "water-former"), with "dephlogisticated air" (air in the process of combustion, at present known to exist oxygen) by electrically sparking mixtures of the gases. All of the researchers noted Cavendish'south production of pure h2o by burning hydrogen in oxygen, only they interpreted the reaction in varying ways inside the framework of phlogiston theory. Lavoisier learned of Cavendish'southward experiment in June 1783 via Charles Blagden (before the results were published in 1784), and immediately recognized water as the oxide of a hydroelectric gas.[43]
In cooperation with Laplace, Lavoisier synthesized water by burning jets of hydrogen and oxygen in a bell jar over mercury. The quantitative results were good enough to back up the contention that water was not an element, every bit had been thought for over 2,000 years, but a compound of two gases, hydrogen and oxygen. The estimation of water every bit a compound explained the inflammable air generated from dissolving metals in acids (hydrogen produced when water decomposes) and the reduction of calces by inflammable air (a combination of gas from calx with oxygen to form water).[xl]
Despite these experiments, Lavoisier'south antiphlogistic arroyo remained unaccepted past many other chemists. Lavoisier labored to provide definitive proof of the composition of water, attempting to apply this in back up of his theory. Working with Jean-Baptiste Meusnier, Lavoisier passed water through a red-hot iron gun barrel, assuasive the oxygen to form an oxide with the atomic number 26 and the hydrogen to sally from the end of the pipage. He submitted his findings of the composition of water to the Académie des Sciences in April 1784, reporting his figures to viii decimal places.[40] Opposition responded to this further experimentation past stating that Lavoisier continued to draw the incorrect conclusions and that his experiment demonstrated the deportation of phlogiston from fe by the combination of water with the metallic. Lavoisier developed a new apparatus which utilized a pneumatic trough, a set up of balances, a thermometer, and a barometer, all calibrated carefully. Xxx savants were invited to witness the decomposition and synthesis of water using this apparatus, disarming many who attended of the correctness of Lavoisier's theories. This demonstration established h2o as a compound of oxygen and hydrogen with great certainty for those who viewed information technology. The dissemination of the experiment, however, proved subpar, every bit it lacked the details to properly display the corporeality of precision taken in the measurements. The newspaper ended with a hasty argument that the experiment was "more sufficient to lay concord of the certainty of the proposition" of the composition of h2o and stated that the methods used in the experiment would unite chemical science with the other physical sciences and accelerate discoveries.[44]
Elementary Treatise of Chemistry

Lavoisier employed the new nomenclature in his Traité élémentaire de chimie (Uncomplicated Treatise on Chemical science), published in 1789. This work represents the synthesis of Lavoisier's contribution to chemistry and can be considered the first modern textbook on the subject. The core of the piece of work was the oxygen theory, and the piece of work became a most effective vehicle for the manual of the new doctrines. It presented a unified view of new theories of chemistry, contained a clear statement of the law of conservation of mass, and denied the existence of phlogiston. This text clarified the concept of an element as a substance that could not exist broken downward by any known method of chemic analysis and presented Lavoisier's theory of the formation of chemic compounds from elements. It remains a classic in the history of science. While many leading chemists of the time refused to accept Lavoisier'due south new ideas, demand for Traité élémentaire equally a textbook in Edinburgh was sufficient to merit translation into English inside almost a year of its French publication.[45] In any event, the Traité élémentaire was sufficiently sound to convince the next generation.
Physiological work

Lavoisier (wearing goggles) operates his solar furnace to prevent contamination from combustion products.
The relationship between combustion and respiration had long been recognized from the essential role which air played in both processes. Lavoisier was about obliged, therefore, to extend his new theory of combustion to include the area of respiration physiology. His first memoirs on this topic were read to the Academy of Sciences in 1777, just his most significant contribution to this field was made in the winter of 1782–1783 in association with Laplace. The result of this work was published in a memoir, "On Heat." Lavoisier and Laplace designed an ice calorimeter appliance for measuring the corporeality of heat given off during combustion or respiration. The outer beat of the calorimeter was packed with snow, which melted to maintain a constant temperature of 0 °C around an inner beat filled with ice. Past measuring the quantity of carbon dioxide and heat produced by confining a live guinea squealer in this appliance, and past comparing the amount of oestrus produced when sufficient carbon was burned in the water ice calorimeter to produce the same amount of carbon dioxide as that which the republic of guinea pig exhaled, they concluded that respiration was, in fact, a irksome combustion procedure. Lavoisier stated, "la respiration est donc une combustion," that is, respiratory gas exchange is a combustion, like that of a candle burning.[46]
This continuous slow combustion, which they supposed took place in the lungs, enabled the living animal to maintain its body temperature above that of its environs, thus accounting for the puzzling phenomenon of animal heat. Lavoisier continued these respiration experiments in 1789–1790 in cooperation with Armand Seguin. They designed an ambitious set of experiments to study the whole process of body metabolism and respiration using Seguin equally a human guinea pig in the experiments. Their piece of work was only partially completed and published considering of the disruption of the Revolution; simply Lavoisier'south pioneering work in this field served to inspire similar enquiry on physiological processes for generations to come.
Legacy

Lavoisier'south key contributions to chemistry were a result of a witting try to fit all experiments into the framework of a single theory. He established the consistent employ of the chemical balance, used oxygen to overthrow the phlogiston theory, and developed a new organisation of chemical nomenclature which held that oxygen was an essential elective of all acids (which later turned out to exist erroneous).
Lavoisier too did early inquiry in concrete chemistry and thermodynamics in joint experiments with Laplace. They used a calorimeter to estimate the heat evolved per unit of carbon dioxide produced, eventually finding the same ratio for a flame and animals, indicating that animals produced energy past a blazon of combustion reaction.
Lavoisier also contributed to early on ideas on composition and chemic changes by stating the radical theory, believing that radicals, which function every bit a unmarried grouping in a chemical procedure, combine with oxygen in reactions. He also introduced the possibility of allotropy in chemical elements when he discovered that diamond is a crystalline form of carbon.
He was as well responsible for the construction of the gasometer, an expensive instrument he used at his demonstrations. While he used his gasometer exclusively for these, he also created smaller, cheaper, more practical gasometers that worked with a sufficient degree of precision that more than chemists could recreate.[47]
He was essentially a theorist, and his peachy merit lay in his chapters to take over experimental work that others had carried out—without always adequately recognizing their claims—and by a rigorous logical procedure, reinforced by his ain quantitative experiments, expounding the truthful explanation of the results.[ commendation needed ] He completed the work of Black, Priestley and Cavendish, and gave a correct explanation of their experiments.
Overall, his contributions are considered the most important in advancing chemical science to the level reached in physics and mathematics during the 18th century.[48]
Awards and honours
During his lifetime, Lavoisier was awarded a golden medal by the Male monarch of French republic for his work on urban street lighting (1766), and was appointed to the French Academy of Sciences (1768).[ten] He was elected as a member of the American Philosophical Society in 1775.[49]
Lavoisier's piece of work was recognized as an International Celebrated Chemical Landmark by the American Chemical Society, Académie des sciences de L'institut de France and the Société Chimique de France in 1999.[50] Antoine Laurent Lavoisier'south Louis 1788 publication entitled Méthode de Nomenclature Chimique, published with colleagues Louis-Bernard Guyton de Morveau, Claude Louis Berthollet, and Antoine François, comte de Fourcroy,[51] was honored by a Citation for Chemical Breakthrough Award from the Division of History of Chemical science of the American Chemical Social club, presented at the Académie des Sciences (Paris) in 2015.[52] [53]

Medal commemorating Franklin and Lavoisier, 2018
A number of Lavoisier Medals have been named and given in Lavoisier'south award, by organizations including the Société chimique de France, the International Lodge for Biological Calorimetry, and the DuPont company[54] [55] [56] He is besides commemorated by the Franklin-Lavoisier Prize, marking the friendship of Antoine-Laurent Lavoisier and Benjamin Franklin. The prize, which includes a medal, is given jointly past the Fondation de la Maison de la Chimie in Paris, France and the Scientific discipline History Institute in Philadelphia, PA, USA.[57] [58]
Selected writings
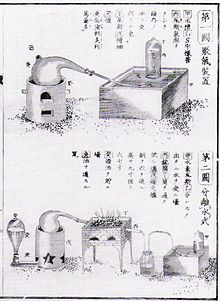
The work of Lavoisier was translated in Nippon in the 1840s, through the procedure of Rangaku. Page from Udagawa Yōan's 1840 Seimi Kaisō
- Opuscules physiques et chimiques (Paris: Chez Durand, Didot, Camaraderie, 1774). (Second edition, 1801)
- L'art de fabriquer le salin et la potasse, publié par ordre du Roi, par les régisseurs-généraux des Poudres & Salpêtres (Paris, 1779).
- Teaching sur les moyens de suppléer à la disette des fourrages, et d'augmenter la subsistence des bestiaux, Supplément à l'teaching sur les moyens de pourvoir à la disette des fourrages, publiée par ordre du Roi le 31 mai 1785 (Instruction on the means of compensating for the food shortage with forage, and of increasing the subsistence of cattle, Supplement to the instruction on the means of providing for the nutrient shortage with forage, published by gild of King on 31 May 1785).
- (with Guyton de Morveau, Claude-Louis Berthollet, Antoine Fourcroy) Méthode de classification chimique (Paris: Chez Cuchet, 1787)
- (with Fourcroy, Morveau, Buck, Baumé, d'Arcet, and Sage) Nomenclature chimique, ou synonymie ancienne et moderne, pour servir à l'intelligence des auteurs. (Paris: Chez Cuchet, 1789)
- Traité élémentaire de chimie, présenté dans un ordre nouveau et d'après les découvertes modernes (Paris: Chez Cuchet, 1789; Bruxelles: Cultures et Civilisations, 1965) (lit. Elementary Treatise on Chemistry, presented in a new order and aslope modern discoveries) as well here
- (with Pierre-Simon Laplace) "Mémoire sur la chaleur [ permanent dead link ] ," Mémoires de l'Académie des sciences (1780), pp. 355–408.
- Mémoire contenant les expériences faites sur la chaleur, pendant l'hiver de 1783 à 1784, par P.Southward. de Laplace & A. K. Lavoisier [ permanent dead link ] (1792)
- Mémoires de physique et de chimie (1805: posthumous)
In translation
- Essays Physical and Chemical (London: for Joseph Johnson, 1776; London: Frank Cass and Company Ltd., 1970) translation by Thomas Henry of Opuscules physiques et chimiques
- The Fine art of Manufacturing Alkali metal Salts and Potashes, Published by Social club of His Nigh Christian Majesty, and canonical by the Royal Academy of Sciences (1784) trans. past Charles Williamos[59] of Fifty'fine art de fabriquer le salin et la potasse
- (with Pierre-Simon Laplace) Memoir on Estrus: Read to the Royal University of Sciences, 28 June 1783, by Messrs. Lavoisier & De La Place of the same University. (New York: Neale Watson Academic Publications, 1982) trans. past Henry Guerlac of Mémoire sur la chaleur
- Essays, on the Effects Produced by Various Processes On Atmospheric Air; With A Item View To An Investigation Of The Constitution Of Acids, trans. Thomas Henry (London: Warrington, 1783) collects these essays:
- "Experiments on the Respiration of Animals, and on the Changes effected on the Air in passing through their Lungs." (Read to the Académie des Sciences, three May 1777)
- "On the Combustion of Candles in Atmospheric Air and in Dephlogistated Air." (Communicated to the Académie des Sciences, 1777)
- "On the Combustion of Kunckel's Phosphorus."
- "On the Existence of Air in the Nitrous Acid, and on the Means of decomposing and recomposing that Acid."
- "On the Solution of Mercury in Vitriolic Acid."
- "Experiments on the Combustion of Alum with Phlogistic Substances, and on the Changes effected on Air in which the Pyrophorus was burned."
- "On the Vitriolisation of Martial Pyrites."
- "General Considerations on the Nature of Acids, and on the Principles of which they are composed."
- "On the Combination of the Matter of Burn with Evaporable Fluids; and on the Germination of Rubberband Aëriform Fluids."
- "Reflections on Phlogiston", translation past Nicholas West. Best of "Réflexions sur le phlogistique, pour servir de suite à la théorie de la combustion et de la calcination" (read to the Académie Royale des Sciences over two nights, 28 June and 13 July 1783). Published in ii parts:
- All-time, Nicholas W. (2015). "Lavoisier's "Reflections on phlogiston" I: Confronting phlogiston theory". Foundations of Chemistry. 17 (2): 361–378. doi:10.1007/s10698-015-9220-v. S2CID 170422925.
- All-time, Nicholas Due west. (2016). "Lavoisier'southward "Reflections on phlogiston" II: On the nature of oestrus". Foundations of Chemistry. 18 (i): 3–13. doi:10.1007/s10698-015-9236-10. S2CID 94677080.
- Method of chymical nomenclature: proposed by Messrs. De Moreau, Lavoisier, Bertholet, and De Fourcroy (1788) Lexicon
- Elements of Chemistry, in a New Systematic Gild, Containing All the Modern Discoveries (Edinburgh: William Creech, 1790; New York: Dover, 1965) translation by Robert Kerr of Traité élémentaire de chimie. ISBN 978-0-486-64624-4 (Dover).
- 1799 edition
- 1802 edition: volume 1, volume 2
- Some illustrations from 1793 edition
- Some more illustrations from the Science History Institute
- More illustrations (from Collected Works) from the Science History Institute
Come across also
- Royal Committee on Animal Magnetism
Notes
- ^ "Lavoisier, Antoine Laurent". Lexico UK English Lexicon. Oxford Academy Press. n.d. Retrieved 30 July 2019.
- ^ "Lavoisier". Collins English Dictionary. HarperCollins. Retrieved xxx July 2019.
- ^ "Lavoisier". Merriam-Webster Dictionary . Retrieved thirty July 2019.
- ^ a b (in French) Lavoisier, le parcours d'united nations scientifique révolutionnaire CNRS (Heart National de la Recherche Scientifique)
- ^ Schwinger, Julian (1986). Einstein'due south Legacy. New York: Scientific American Library. p. 93. ISBN978-0-7167-5011-6.
- ^ In his table of the elements, Lavoisier listed five "salifiable earths" (i.east., ores that could exist made to react with acids to produce salts (salis = table salt, in Latin)): chaux (calcium oxide), magnésie (magnesia, magnesium oxide), baryte (barium sulfate), alumine (alumina, aluminum oxide), and silice (silica, silicon dioxide). About these "elements", Lavoisier speculates: "We are probably just acquainted as however with a part of the metallic substances existing in nature, as all those which have a stronger affinity to oxygen than carbon possesses, are incapable, hitherto, of existence reduced to a metallic country, and consequently, beingness only presented to our ascertainment under the form of oxyds, are confounded with earths. It is extremely probable that barytes, which nosotros have only now bundled with earths, is in this state of affairs; for in many experiments it exhibits backdrop virtually approaching to those of metal bodies. It is even possible that all the substances we telephone call earths may be only metal oxyds, irreducible by any hitherto known process." – from p. 218 of: Lavoisier with Robert Kerr, trans., Elements of Chemistry, ..., quaternary ed. (Edinburgh, Scotland: William Creech, 1799). (The original passage appears in: Lavoisier, Traité Élémentaire de Chimie, ... (Paris, France: Cuchet, 1789), vol. 1, p. 174.)
- ^ Schama, Simon (1989). Citizens: A Chronicle of the French Revolution. Alfred A Knopf. p. 73.
- ^ Herbermann, Charles, ed. (1913). . Catholic Encyclopedia. New York: Robert Appleton Company.
- ^ Chisholm, Hugh, ed. (1911). . Encyclopædia Britannica. Vol. sixteen (11th ed.). Cambridge Academy Press. p. 295.
- ^ a b Yount, Lisa (2008). Antoine Lavoisier : founder of modern chemistry . Berkeley Heights, NJ: Enslow Publishers. p. 115. ISBN978-0-7660-3011-iv . Retrieved 25 July 2016.
- ^ a b c d Duveen, Dennis I. (1965). Supplement to a bibliography of the works of Antoine Laurent Lavoisier, 1743–1794. London: Dawsons.
- ^ a b c McKie, Douglas (1935). Bibliographic Details Antoine Lavoisier, the father of mod chemical science, past Douglas McKie ... With an introduction by F.G. Donnan. London: Five. Gollancz ltd.
- ^ a b c d due east f Bibliographic Details Lavoisier in perspective / edited by Marco Beretta. Munich: Deutsches Museum. 2005.
- ^ a b c d e f yard Bong, Madison Smart (2005). Lavoisier in the yr one . New York: West.W. Norton.
- ^ McKie, Douglas (1952). Antoine Lavoisier: scientist, economist, social reformer . New York: Schuman.
- ^ Citizens, Simon Schama. Penguin 1989 p. 236
- ^ Eagle, Cassandra T.; Jennifer Sloan (1998). "Marie Anne Paulze Lavoisier: The Mother of Modernistic Chemistry". The Chemic Educator. 3 (v): 1–18. doi:10.1007/s00897980249a. S2CID 97557390.
- ^ Donovan, Arthur (1996). Antoine Lavoisier: Science, Administration, and Revolution. Cambridge: Cambridge University Printing. p. 273. ISBN978-0-521-56672-8.
- ^ Jean-Pierre Poirier (1998). Lavoisier: Pharmacist, Biologist, Economist . University of Pennsylvania Press. pp. 24–26. ISBN978-0-8122-1649-3.
- ^ a b W.R. Aykroyd (12 May 2014). Three Philosophers: Lavoisier, Priestley and Cavendish. Elsevier Scientific discipline. pp. 168–170. ISBN978-1-4831-9445-v.
- ^ Arthur Donovan (xi April 1996). Antoine Lavoisier: Science, Administration and Revolution. Cambridge University Press. pp. 123–125. ISBN978-0-521-56672-eight.
- ^ Citizens, Simon Schama, Penguin 1989 p. 313
- ^ a b Dutton, William Southward. (1942), Du Pont: I Hundred and 40 Years, Charles Scribner's Sons, LCCN 42011897.
- ^ Chronicle of the French Revolution, Jacques Legrand, Longman 1989, p. 216
- ^ Companion to the French Revolution, John Paxton, Facts on File Publications 1988, p. 120
- ^ a b A Cultural History of the French Revolution, Emmet Kennedy, Yale Academy Press 1989, p. 193
- ^ Relate of the French Revolution, Jacques Legrand, Longman 1989, p. 204
- ^ a b Chronicle of the French Revolution, Jacques Legrand, Longman 1989, p. 356
- ^ O'Connor, J.J.; Robertson, Due east.F. (26 September 2006). "Joseph-Louis Lagrange". Archived from the original on 2 May 2006. Retrieved 20 April 2006.
In September 1793 a law was passed ordering the arrest of all foreigners born in enemy countries and all their property to be confiscated. Lavoisier intervened on behalf of Lagrange, who certainly fell under the terms of the law. On eight May 1794, afterward a trial that lasted less than a 24-hour interval, a revolutionary tribunal condemned Lavoisier and 27 others to death. Lagrange said on the death of Lavoisier, who was guillotined on the afternoon of the day of his trial
- ^ Chronicle of the French Revolution, Longman 1989 p. 202
- ^ "Today in History: 1794: Antoine Lavoisier, the father of mod chemistry, is executed on the guillotine during France's Reign of Terror". Archived from the original on 15 June 2013.
- ^ Commenting on this quotation, Denis Duveen, an English language expert on Lavoiser and a collector of his works, wrote that "information technology is pretty certain that it was never uttered". For Duveen's show, see the post-obit: Duveen, Denis I. (February 1954). "Antoine Laurent Lavoisier and the French Revolution". Journal of Chemical Educational activity. 31 (2): 60–65. Bibcode:1954JChEd..31...60D. doi:10.1021/ed031p60. .
- ^ Delambre, Jean-Baptiste (1867). (in French). Gauthier-Villars. 15–57 – via Wikisource.
- ^ Guerlac, Henry (1973). Antoine-Laurent Lavoisier – Chemist and Revolutionary. New York: Charles Scribner'due south Sons. p. 130.
- ^ (In French) Yard.-A. Paulze, épouse et collaboratrice de Lavoisier, Vesalius, VI, 2, 105–113, 2000, p. 110.
- ^ a b Lavoisier, Antoine (1777) "Mémoire sur la combustion en général" Archived 17 June 2013 at the Wayback Machine ("On Combustion in General"). Mémoires de fifty'Académie des sciences. English translation
- ^ Petrucci R.H., Harwood W.S. and Herring F.K., Full general Chemistry (8th ed. Prentice-Hall 2002), p. 34
- ^ "An Historical Annotation on the Conservation of Mass".
- ^ Duveen, Denis; Klickstein, Herbert (September 1954). "The Introduction of Lavoisier'south Chemical Classification into America". The History of Science Club. 45 (3).
- ^ a b c d Golinski, Jan (1994). "Precision instruments and the demonstrative gild of proof in Lavoisier'southward chemistry". Osiris. 9: 30–47. doi:10.1086/368728. JSTOR 301997. S2CID 95978870.
- ^ Kirwan, Essay on Phlogiston, viii, eleven.
- ^ Lavoisier, Antoine (1778) "Considérations générales sur la nature des acides" Archived 17 June 2013 at the Wayback Machine ("General Considerations on the Nature of Acids"). Mémoires de 50'Académie des sciences. lavoisier.cnrs.fr
- ^ Gillispie, Charles Coulston (1960). The Edge of Objectivity: An Essay in the History of Scientific Ideas. Princeton University Press. p. 228. ISBN0-691-02350-half dozen.
- ^ Lavoisier and Meusnier, "Développement" (cit. northward. 27), pp. 205–209; cf. Holmes, Lavoisier (cn. eight), p. 237.
- ^ Come across the "Advertizing," p. vi of Kerr'due south translation, and pp. xxvi–xxvii, xxviii of Douglas McKie's introduction to the Dover edition.
- ^ Is a Calorie a Calorie? American Journal of Clinical Nutrition, Vol. 79, No. v, 899S–906S, May 2004
- ^ Levere, Trevor (2001). Transforming Matter. Maryland: The Johns Hopkins University Printing. pp. 72–73. ISBN978-0-8018-6610-4.
- ^ Gillespie, Charles C. (1996), Foreword to Lavoisier past Jean-Pierre Poirier, University of Pennsylvania Printing, English Edition.
- ^ "APS Member History". search.amphilsoc.org . Retrieved 28 May 2021.
- ^ "Antoine-Laurent Lavoisier: The Chemic Revolution". National Celebrated Chemical Landmarks. American Chemic Society. Archived from the original on 23 Feb 2013. Retrieved 25 March 2013.
- ^ Guyton de Morveau, Louis Bernard; Lavoisier, Antoine Laurent; Berthollet, Claude-Louis; Fourcroy, Antoine-François de (1787). Méthode de Nomenclature Chimique. Paris, France: Chez Cuchet (Sous le Privilége de l'Académie des Sciences).
- ^ "2015 Awardees". American Chemical Lodge, Partition of the History of Chemistry. University of Illinois at Urbana-Champaign School of Chemical Sciences. 2015. Retrieved ane July 2016.
- ^ "Citation for Chemic Quantum Award" (PDF). American Chemical Society, Sectionalisation of the History of Chemistry. University of Illinois at Urbana-Champaign School of Chemical Sciences. 2015. Retrieved ane July 2016.
- ^ "Société Chimique de France". www.societechimiquedefrance.fr. Archived from the original on 29 March 2019. Retrieved 28 March 2019.
- ^ "International Lodge for Biological Calorimetry (ISBC) - Nigh ISBC_". biocalorimetry.ucoz.org . Retrieved 28 March 2019.
- ^ workflow-procedure-service. "The Lavoisier Medal honors exceptional scientists and engineers | DuPont Us". world wide web.dupont.com . Retrieved 28 March 2019.
- ^ "Le Prix Franklin–Lavoiser2018 a été décerné au Comité Lavoisier". La Gazette du Laboratoire. 20 June 2018. Retrieved 15 January 2019.
- ^ "Franklin-Lavoisier Prize". Science History Establish. Archived from the original on 26 March 2020. Retrieved 26 March 2020.
- ^ See Denis I. Duveen and Herbert Southward. Klickstein, "The "American" Edition of Lavoisier's L'art de fabriquer le salin et la potasse," The William and Mary Quarterly, 3rd Series 13:4 (October 1956), 493–498.
Farther reading
- Herbermann, Charles, ed. (1913). . Catholic Encyclopedia. New York: Robert Appleton Company.
- Bailly, J.-S., "Secret Written report on Mesmerism or Animal Magnetism", International Journal of Clinical and Experimental Hypnosis, Vol. 50, No. four, (Oct 2002), pp. 364–368. doi:10.1080/00207140208410110
- Berthelot, Yard. (1890). La révolution chimique: Lavoisier. Paris: Alcan.
- Catalogue of Printed Works past and Memorabilia of Antoine Laurent Lavoisier, 1743–1794... Exhibited at the Grolier Social club (New York, 1952).
- Daumas, Thou. (1955). Lavoisier, théoricien et expérimentateur. Paris: Presses Universitaires de France.
- Donovan, Arthur (1993). Antoine Lavoisier: Scientific discipline, Administration, and Revolution. Cambridge, England: Cambridge University Press.
- Duveen, D.I. and H.S. Klickstein, A Bibliography of the Works of Antoine Laurent Lavoisier, 1743–1794 (London, 1954)
- Franklin, B., Majault, One thousand.J., Le Roy, J.B., Sallin, C.L., Bailly, J.-S., d'Arcet, J., de Bory, G., Guillotin, J.-I. & Lavoisier, A., "Report of The Commissioners charged by the King with the Examination of Animal Magnetism", International Periodical of Clinical and Experimental Hypnosis, Vol.50, No.4, (Oct 2002), pp. 332–363. doi:ten.1080/00207140208410109
- Greyness, Vivian (1982). The Chemist Who Lost His Caput: The Story of Antoine Lavoisier . Coward, McCann & Geoghegan, Inc. ISBN9780698205598.
- Gribbin, John (2003). Scientific discipline: A History 1543–2001. Gardners Books. ISBN978-0-14-029741-half-dozen.
- Guerlac, Henry (1961). Lavoisier – The Crucial Year . Ithaca, New York: Cornell University Press.
- Holmes, Frederic Lawrence (1985). Lavoisier and the Chemistry of Life. Madison, Wisconsin: University of Wisconsin Press.
- Holmes, Frederic Lawrence (1998). Antoine Lavoisier – The Side by side Crucial Year, or the Sources of his Quantitative Method in Chemistry. Princeton Academy Press.
- Jackson, Joe (2005). A World on Fire: A Heretic, An Blueblood And The Race to Detect Oxygen. Viking.
- Johnson, Horton A. (2008). "Revolutionary Instruments, Lavoisier's Tools as Objets d'Art". Chemical Heritage Magazine. 26 (1): 30–35.
- Kelly, Jack (2004). Gunpowder: Alchemy, Bombards, & Pyrotechnics. Basic Books. ISBN978-0-465-03718-6.
- McKie, Douglas (1935). Antoine Lavoisier: The Father of Modernistic Chemistry. Philadelphia: J.P. Lippincott Visitor.
- McKie, Douglas (1952). Antoine Lavoisier: Scientist, Economist, Social Reformer . New York: Henry Schuman.
- Poirier, Jean-Pierre (1996). Lavoisier (English ed.). University of Pennsylvania Press.
- Scerri, Eric (2007). The Periodic Table: Its Story and Its Significance . Oxford University Printing. ISBN978-0-19-530573-ix.
- Smartt Bell, Madison (2005). Lavoisier in the Yr Ane: The Birth of a New Science in an Age of Revolution . Atlas Books, W.Due west. Norton.
External links
- Archives: Fonds Antoine-Laurent Lavoisier, Le Comité Lavoisier, Académie des sciences
- Panopticon Lavoisier a virtual museum of Antoine Lavoisier
- Bibliography at Panopticon Lavoisier
- Les Œuvres de Lavoisier
- About his piece of work
- Location of Lavoisier's laboratory in Paris
- Radio four program on the discovery of oxygen past the BBC
- Who was the beginning to classify materials as "compounds"? – Fred Senese
- Cornell Academy's Lavoisier collection
- His writings
- Works past Antoine Lavoisier at Project Gutenberg
- Works past or about Antoine Lavoisier at Internet Archive
- Les Œuvres de Lavoisier (The Complete Works of Lavoisier) edited by Pietro Corsi (Oxford University) and Patrice Bret (CNRS) (in French)
- Oeuvres de Lavoisier (Works of Lavoisier) at Gallica BnF in six volumes. (in French)
- WorldCat author page
- Title page, woodcuts, and copperplate engravings past Madame Lavoisier from a 1789 showtime edition of Traité élémentaire de chimie (all images freely available for download in a variety of formats from Science History Institute Digital Collections at digital.sciencehistory.org.
Source: https://en.wikipedia.org/wiki/Antoine_Lavoisier
0 Response to "what new information did antoine lavoisier contribute to the understanding of the atom"
Postar um comentário